PUBLISHED IN 02.02.2023
Brassicaceae is a remarkable family composed mainly of herbs from temperate (rarely in tropical mountainous regions), being quite diverse in the region of East Asia, the Mediterranean basin, Central Asia and North America. Plants of the World On Line (POWO) recognizes 345 genera in Brassicaceae (SEE), while BrassiBase, a platform dedicated exclusively to the family, points to 351 genera, with 3,977 spp. within 52 tribes (SEE). The family is marked by an exorbitant amount of naturalized, cultivated and invasive species in various parts of the world, so the vast majority of local treatments of the group contain many citations of non-native species and genera, at various scales.
In terms of native diversity, in addition to the regions of the northern hemisphere already mentioned, it is worth mentioning interesting radiations from the Andean and Patagonian region of South America, South Africa and Australia, all with many endemic genera. Few genera occur in tropical zones, most restricted to mountainous regions. In Africa, for example, few genera colonized the African highlands, to mention prominently Arabis, Arabidopsis, Crambe, Diceratella, Oreophyton, Sisymbrium, Subularia, Mummenhoffia and Turritis; tribe Microlepidieae (16/55) has a extraordinary radiation in Australia region; in southern Africa, three near restricted genera occur: Aplanodes, Chamira and Heliophila, the latter with c. 100 spp., very distinct morphologically.
Morphologically, Brassicaceae are mostly delicate herbs, but there are a lot of sessile rosettes, dendroid rosettes and succulents, aquatic herbs, large shrubs, small arctic tundra herbs, thorny shrubs from North Africa, some rare lianas, nanoplants from snow line and high mountains, but saplings (or larger), woody lianas and epiphytes are virtually absent. The Brassicaceae are easily distinguished by the cruciform corolla and tetradynamous stamens. The exceptions are in some species of Lepidium that have only two stamens and a rudimentary or absent corolla.
In New World there are c. 1,170 spp., c. 400 in South America; the only native Neotropical crop is Lepidium meyenii (maca) cultivated in the high Peruvian Andes and consumed locally.
There is almost no academic work focused on the family in Brazil, which is surprising given that the family represents one of the most interesting and explored in the botanical literature of many neighboring countries. With the objective of creating a point of support in this discussion, NSAA made a review of Brassicaceae in Brazil, using as reference the species cited as Brazilian in POWO (SEE), each one being analyzed within the scope of the VPA and the records of each one. in Species Link (SEE), with eventual complement from other sources, such as Zuloaga et al. (Darwiniana, 2019). Maps were edited based on these records, one per genus, and some referenced images were added. In some genera, we excluded some records due to the fact that they are too disjoint from the occurrence bulk of a given species, suggesting that they are material incorrectly identified, or non-native material.
In this analysis we identify 4 genera and 11 spp. native in Brazil, two endemics, both known only the type collection.
TAXONOMY
Brassicaceae includes two subfamilies, Aethionemoideae (1/58) and Brassicoidae (remaining), the latter with five subtribes, Arabodae (117/c. 1,190, Arabideae, Alysseae, Asperuginoideae, Stevenieae), at South America with 84 spp. in two genera from Venezuela to Argentina and Chile; Heliophilodae (22/c. 111, Anastaticeae, Asteae, Biscutelleae, Chamireae, Cremolobeae, Eudemeae, Heliophileae, Hillielleae, Iberideae, Megacarpaeeae Notothlaspideae, Schizopetaleae, Subularieae) from S and E Europe, Mediterranean, W Asia, Mexico, Himalayas, Siberia, Russian Far East, Alaska, Yukon and South America; and Hesperodae (Eurasia, Anchonieae, Buniadeae, Chorisporeae, Dontostemoneae, Euclidieae, Hesperideae, Shehbazieae) does not occur in Brazil.
Andrzeiowskia (1, SE Europe, SW Asia), Raphanorhyncha (1, Mexico), Veselskya (1, Afghanistan) are unplaced Brassicaceae.
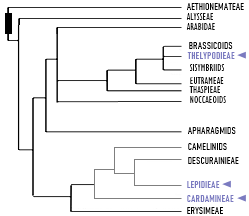
|
| ADAPTED PHYLOGENY OF BRASSICACEAE WITH EMPHASIS ON CLADES WITH NATIVE BRAZILIAN SPECIES |
1. SUPERTRIBE CAMELINODAE
16 lineages, Alyssopsideae, Arabidopsideae, Boechereae, Beilstein, Camelineae, Cardamineae, Crucihimalayeae, Descurainieae, Erysimeae, Halimolobeae, Hemilophieae, Lepidieae, Malcolmieae, Microlepidieae, Oreophytoneae, Physarieae, Smelowskieae, Turritideae, Yinshanieae, 11 of them absent in South America. For South Americans, tribes Descuiraneae (6/c. 45, 12 spp. in three genera from Venezuela to Argentina and Chile), Physarieae (7/133, only six sp. of Physaria (Nutt. ex. Torr. & A. Gray) A. Gray. from Bolivia, Argentina and Uruguay) and Halimolobeae (6/39, 13 spp. in 4 genera from Venezuela to Argentina and Chile) does not occur in Brazil. Brazilian members belogs tribes Cardamineae and Lepidieae.
1.1 Tribe Cardamineae - c. 12 genera and c. 335 spp., almost all from northern hemisphere south up to Mexico except Aplanodes (2) in South Africa and Lesotho, and two subcosmopolitan, Rorippa and Cardamine.
Cardamine L.
Annuals, biennials, or perennials, usually rhizomatous, sometimes tuberiform, stoloniferous or caudex; not scapose, often subscapose; glabrous or pubescent. Stems erect, ascending, decumbent, or prostrate, unbranched or branched. Leaves: cauline, rhizomal, or basal; rhizomal and basal rosulate or not, petiolate, blade margins entire, toothed, or 1-3-pinnatisect, or palmately lobed, sometimes trifoliolate, pinnately, palmately, or bipinnately compound (leaflets petiolulate, subsessile, or sessile); cauline (usually alternate, rarely opposite or whorled) petiolate or sessile, blade (base cuneate, attenuate, or auriculate to sagittate), margins entire, dentate, or variously lobed, (leaflets petiolulate or sessile). Racemes (corymbose or paniculate, bracteate in C. pattersonii), elongated in fruit. Fruiting pedicels erect, ascending, divaricate, or reflexed, slender or stout. Flowers: sepals (caducous), usually erect, rarely spreading or ascending, ovate or oblong, lateral pair saccate or not basally, (usually glabrous, rarely pubescent); petals (rarely absent), white, pink, purple, or lilac, obovate, spatulate, or oblanceolate, claw absent or strongly differentiated from blade, (apex obtuse, rounded, emarginate, or subemarginate); stamens (6, rarely 4), equal in length; filaments not dilated basally; anthers ovate, oblong, or linear, (apex obtuse), glabrous [rarely pubescent]; nectar glands confluent, lateral glands annular or semiannular, subtending bases of stamens, median glands present (2, rarely 4) or absent. Fruits siliques, sessile, usually linear, rarely narrowly oblong or narrowly lanceolate, smooth or torulose, latiseptate; valves (papery, elastically dehiscent, becoming spirally or circinately coiled) each not veined, glabrous or, rarely, pubescent; replum strongly flattened; septum complete, (membranous); ovules 4-80 per ovary; style usually distinct, rarely obsolete; stigma capitate. Seeds uniseriate, flattened, usually not winged, rarely margined or winged, oblong, ovoid, or globose; seed coat (smooth, minutely reticulate, colliculate, or rugose) mucilaginous or not when wetted; cotyledons accumbent, rarely incumbent. x = 7, 8 (FNA).
DISTRIBUITON 260 spp., mainly restricteds for a single landmass region: C & E Asia from E Siberia to Philipppines (c. 65 spp.), northern Eurasia from Azores to Turkey and Siberia (c. 50 spp.), Arctic Canada to Central America and Caribbean (c. 40 spp., C. ocoana O.E.Schulz endemic to Dominican Republic), Australia to New Zealand and adjacent islands (c. 55 spp.), South America (27 exclusive spp.); tropical Africa and Middle East has only one exclusive species each; Australasia from Indonesia to New Caledonia has only three exclusive species (New Guinea and Java with endemics).
14 non South American spp. extrapolate this pattern, being seven spp. from Eurasia up to North America; C. trichocarpa Hochst. ex A.Rich. from tropical Africa to India; C. macrophylla Willd. and C. impatiens L. from Europe to tropical China and India; C. hirsuta L. and C. flexuosa With. from Europe and tropical Africa to Middle East or New Guinea; C. pratensis L. and C. bellidifolia L. in northern Hemisphere up to U.S.A., Ethiopia and Tibet.
South American has 33 spp., 27 exclusives, C. obliqua Hochst. ex A.Rich. and C. africana L. are amphitropical, native from tropical Africa and from Mexico to Ecuador, the latter also in southern Asia, Australasia and S & SE South America inc. Brazil; C. ovata Benth., C. jamesonii Hook., C. bonariensis Pers. and C. fulcrata Greene occur from Central America, Caribbean or Mexico to South America. For 27 South American exclusives, VPA reconizes only 19. Endemic species in South America occur in Bolivia (2), Chile (6), Colombia (1), Ecuador (3), Uruguay (1, C. subterranea Larrañaga) and Venezuela (1).
1. Cardamine africana L. ‣ subpantropical species, from tropical & S Africa, Yemen, India to W Malesia, Guatemala to Ecuador, Bolivia, Argentina and S & SE Brazil. In Brazil occur in small places in Minas Gerais, Rio de Janeiro, São Paulo, Paraná, Santa Catarina and Rio Grande do Sul states.
2. Cardamine bonariensis Pers. ‣ widely distributed from Mexico to Argentina and S, SE & NE Brazil; widely in Brazil from Paraíba to Rio Grande do Sul state, with a gap between Bahia and Minas Gerais.
3. Cardamine chenopodiifolia Pers. ‣ collected from Bolivia, Argentina, Paraguay and E & SE Brazil. In Brazil is widely from Minas Gerais to Rio Grande do Sul state, with a disjunct record near Bahia state.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
 |
| Cardamine bonariensis Pers. in Santa Catarina state, Brazil © Species Link/Fototeca Paulo Schwirkowski – FPS |
 |
| Cardamine chenopodiifolia Pers. in Uruguay © floranativadeuruguay.blogspot.com.br |
2. Rorippa Scop.
Annuals, biennials, or perennials; (usually aquatic or of mesic habitats, rhizomatous, sometimes with caudex); not scapose; glabrous or pubescent. Stems erect or prostrate, unbranched or branched. Leaves basal and/or cauline; petiolate or sessile; basal (usually withered early), rosulate or not, petiolate, blade margins entire, dentate, sinuate, lyrate, pectinate, or 1-3-pinnatisect; cauline petiolate or sessile, blade (base cuneate, attenuate, auriculate, or sagittate), margins entire, dentate, pinnatifid, or pinnatisect. Racemes slightly to considerably elongated in fruit. Fruiting pedicels erect, suberect, ascending, horizontal, reflexed, or divaricate, usually slender. Flowers: sepals (rarely persistent), erect or spreading, ovate or oblong, lateral pair not or, rarely, saccate basally, (margins often membranous); petals (rarely vestigial or absent), often yellow, sometimes white or pink, usually obovate, spatulate, oblong, or oblanceolate, rarely linear, claw undifferentiated or not from blade, (often shorter than sepals, apex obtuse or emarginate); stamens usually tetradynamous, rarely 4 and equal; anthers usually ovate or oblong, rarely linear, (apex usually obtuse, rarely apiculate); nectar glands confluent, often subtending bases of stamens, median present. Fruits siliques or silicles, usually sessile, rarely shortly stipitate, linear, oblong, ovoid, ellipsoid, pyriform, subglobose, or globose, smooth or torulose, terete or slightly latiseptate; valves papery or leathery, each obscurely veined, glabrous or pubescent; replum (visible), rounded; septum usually complete, rarely perforate; ovules [10-]18-242[-300] per ovary; style obsolete or distinct; stigma capitate, (rarely slightly 2-lobed). Seeds usually biseriate, rarely uniseriate, plump, not or, rarely, winged, oblong, ovoid, ovate, orbicular, cordiform, subglobose, or globose; seed coat (reticulate, colliculate, rugose, tuberculate, or foveolate), mucilaginous or not when wetted; cotyledons accumbent (FNA).
DISTRIBUTION 85 spp., mainly exclusives from a single regiions: Europe inc. Caucasus (13), tropical Africa (3), Pacific islands from Indonesia to Solomon Is. (5, inc. endemics in New Guinea, Java and New Caledonia), Arctic Canada to Costa Rica and Caribbean (20), Australia (4), New Zealand (1), Iran (1), Madagascar and region (2), Marocco (3), S, E & SE Asia (7). Mixed pattern includes Eurasia to Canadá, Australia to New Zealand, Africa to India, Africa to Madagascar, India to Timor one spp. each. 5 spp. are widely distributed in Eurasia; R. indica (L.) Hiern. occur in almost all parts of Old World; R. palustris (L.) Besser occur in over all nothern Hemisphere up to Nicaragua, Sudan and New Guinea.
All 13 South American members by POWO (SEE) are exclusive from continent except Rorippa pinnata (Sessé & Moc.) Rollins, disjuntc from Colombia and Mexico: R. austroamericana Mart.-Laborde (Argentina, Bolivia), R. beckii Al-Shehbaz (Peru, Bolivia), R.bonariensis (Poir.) Macloskie (Ecuador, Peru, Argentina, Paraguay, Brazil), R. clandestina (Spreng.) J.F. Macbr. (Ecuador to Argentina and Brazil), R. coxii (Phil.) L.E. Navas (Chile), R. chubutica (O.E.Schulz) Mart.-Laborde (Argentina and Chile), R. eggersii (O.E. Schulz) J.F. Macbr. (Colombia and Ecuador), R. hilariana (Walp.) Cabrera (Bolivia, Argentina, Paraguay, Brazil), R. mandonii (E. Fourn.) Mart.-Laborde (Colombia to Bolivia and Argentina), R. nana (Schltdl.) J.F. Macbr. (Colombia to Bolivia, S Brazil), R. philippiana (Speg.) Macloskie (Chile) and R. ventanensis Boelcke (Argentina).
VPA recognizes 4 spp. absents in POWO for South America (SEE): R. sarmentosa (Sol. ex G. Forst.) J.F. Macbr., R. palustris (L.) Besser, R. burkartii (Mart.-Laborde) Al-Shehbaz and R. amphibia (L.) Besser; and excludes 3: R. chubutica (O.E.Schulz) Mart.-Laborde, R. pinnata (Sessé & Moc.) Rollins and R. ventanensis Boelcke.
IN BRAZIL Salariato & Zuloaga (Darwiniana, 2020) cites R. bonariensis (Poir.) Macloskie, R. burkartii (Mart.-Laborde) Al-Shehbaz, R. clandestina (Spreng.) J.F. Macbr., R. coxii (F. Phil. ex Phil.) L.E. Navas, R. hilariana (Walp.) Cabrera for Brazil; POWO cites all except R. burkartii and R. coxii, plus R. nana (Schltdl.) J.F. Macbr. (SEE); VPA cites the same all from POWO; Reflora cites all from POWO except R. nana (SEE Reflora). By Species Link, only the three of the Reflora has registered records, and they are the only ones accepted here.
1. Rorippa bonariensis (Poir.) Macloskie. ‣ Ecuador, Peru, Argentina, Paraguay and S Brazil; among the latter country, this species occur only in Rio Grande do Sul state.
2. Rorippa clandestina (Spreng.) J.F. Macbr. ‣ range from Ecuador to Argentina and S Brazil. In Brazil this species ocur in few locations in Paraná, Minas Gerais and Santa Catarina states.
3. Rorippa hilariana (Walp.) Cabrera. ‣ known from Bolivia, Argentina, Paraguay and S Brazil, where occur only in Paraná and Rio Grande do Sul state.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
 |
| Rorippa clandestina (Spreng.) J.F. Macbr. in Santa Catarina state, Brazil © Species Link/Fototeca Paulo Schwirkowski – FPS |
1.2 Tribe Lepidieae - a single genus, Lepidium., recenty incluing Cyphocardamum from Afghanisthan and Lithodraba from Argentina.
C. Lepidium L.
Herbs not scapose; glabrous, pubescent, hirsute, or pilose. Stems usually erect or ascending, sometimes procumbent, decumbent, or prostrate, unbranched or branched. Leaves usually basal and cauline; petiolate or sessile; basal rosulate or not, petiolate (or petiole undifferentiated from blade), blade margins entire, dentate, denticulate, serrate, crenate, or lobed; cauline petiolate or sessile, blade (base auriculate or not), margins entire, dentate, or pinnately divided. Racemes (usually corymbose), elongated or not in fruit. Fruiting pedicels erect to divaricate, slender or stout. Flowers: sepals (usually deciduous, sometimes persistent), usually ovate or oblong, rarely suborbicular; petals (erect or spreading, sometimes rudimentary or absent), obovate, spatulate, oblong, oblanceolate, orbicular, linear, or filiform, claw absent or differentiated from blade, (apex obtuse, rounded, or emarginate); stamens 2 or 4 and equal in length, lateral or median, or 6 and tetradynamous; filaments not dilated basally; anthers ovate or oblong; (ovary placentation apical); nectar glands (4 or 6), distinct, median glands often present. Fruits schizocarps or silicles, (rarely indehiscent), sessile, didymous, oblong, ovate, obovate, cordate, obcordate, elliptic, orbicular, ovoid, obovoid, or globose, strongly angustiseptate or inflated and terete; valves each with prominent veins or not veined, (keeled or rounded, apex winged or not, thin or strongly thickened and ornamented, enclosing or readily releasing seed), glabrous or pubescent; replum rounded, (visible); septum complete or perforated; style absent, obsolete, or distinct, (included or exserted from apical notch); stigma capitate, usually entire, rarely 2-lobed. Seeds oblong or ovate [obovate], plump or flattened, winged, margined, or not winged; seed coat (smooth, minutely reticulate, or papillate), usually copiously mucilaginous when wetted, rarely not; cotyledons usually incumbent (FNA).
DISTRIBUTION subcosmopolitan.
IN BRAZIL Salariato & Zuloaga (Darwiniana, 2020) cites L. auriculatum Regel & Körn., L. bonariense L. and L. didymum L. in Brazil; VPA cites exactly these three species; POWO (SEE) lists these same three for country; Reflora cites only L. auriculatum Regel & Körn. (as L. aletes J.F.Macbr.) and L. bonariense L. (SEE Reflora).
1. Lepidium auriculatum Regel & Körn. ‣ Peru to S Brazil, Argentina, Chile and Uruguay.
2. Lepidium bonariense L. ‣ collected from Bolivia to SE & S. Brazil, Paraguay, Argentina, Chile and Uruguay.
3. Lepidium didymum L. ‣ native from Peru to S Brazil, Argentina, Chile and Uruguay.
4. Lepidium grandifructum C.L.Hitchc. ‣ known only two collections, endemic to Brazil, known only the type collection from Upper Rio Negro Basin in Paraná state.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.

|
Lepidium auriculatum Regel & Korn. in Santa Catarina state, Brazil © Species Link/Fototeca Paulo Schwirkowski – FPS |
 |
| Lepidium bonariense L. in Uruguay © floranativadeuruguay.blogspot.com.br |
2. SUPERTRIBE BRASSICODAE
14 tribes, Aphragmeae, Brassiceae, Calepineae, Coluteocarpeae, Conringieae, Eutremeae, Fourraeeae, Isatideae, Kernereae, Plagiolobeae, Schrenkielleae, Sisymbrieae, Thelypodieae, Thlaspideae, 11 absents in South America and three in continent: Coluteocarpeae (3/127), with Noccaea magelanica (Comm. ex Poir.) Holub in Argentina and Chile, Brassiceae (c. 36/c. 275), with Cakile lanceolata (Willd.) O.E. Schulz in Colombia and Venezuela, and the Brazilian Thelypodieae (28/260), widely distributed and exclusively from New World except Pringlea from Prince Edward, Crozet, Kerguélen, and Heard islands.
D. Polypsecadium O.E.Schulz.
Annual or perennial herbs, subshrubs, or shrubs; stems erect to ascending, branched above; basal leaves absent; racemes many flowered, lax, ebracteate, corymbose, elongated considerably in fruit; petals white to lavender or purple, obovate to spatulate, apex obtuse.
DISTRIBUTION 15 spp., two in N Andes from Colombia and Ecuador, and remaind 12 from Peru to S Argentina (centre of diversity), and one endemic to southern Brazil.
IN BRAZIL Polypsecadium is a very enigmatic genus not recognized in Brazil by the VPA or by Reflora, and has been neglected over the years by scholars in the area.
1. Polypsecadium brasiliense (O.E.Schulz) Al-Shehbaz. ‣ known only the type collection, from Santa Catarina state, no precise location nor collection environment details.
xxx.
