⋵: 'ENDEMIC', IN SPECIES REFERENCE
|
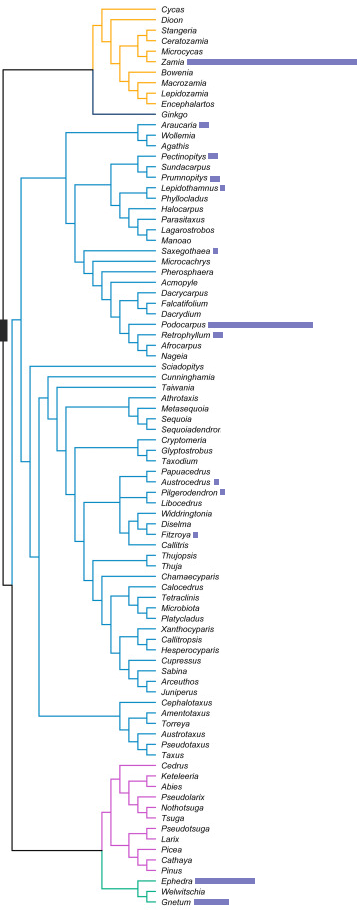
| PHYLOGENY OF GYMNOSPERMS, BASED ON YANG, Y. ET AL. (PLANT DIVERSITY, 2022), WITH EMPHASIZATION ON SOUTH AMERICAN'S (BLUE BARS ARE PROPORTIONAL TO THEIR DIVERSITY ON THE CONTINENT) |
| EPHEDRACEAE | ZAMIACEAE | GNETHACEAE | ARAUCARIACEAE | CUPRESSACEAE | PODOCARPACEAE | G/SPP.(ENDEMICS) | |
| FRENCH GUIANA | 3 | (1/)3 | |||||
| SURINAME | 2 | (1/)2 | |||||
| GUYANA | 4 | (1/)3 | 2/7 | ||||
| VENEZUELA | 4 | 7 | (4/)15(1⋵) | (4/)26 (1⋵) | |||
| COLOMBIA | 26(18⋵) | 6 | (4/)8 | (6/)40 (18⋵) | |||
| ECUADOR | 2 | 4 (1⋵) | 1 | (4/)9 | (7/)16 (1⋵) | ||
| PERU | 3 | 8 (2⋵) | 2 | (4/)10 | (7/)23 (2⋵) | ||
| BOLIVIA | 4 | 2 | 2 | (3/)10 (1⋵) | (6/)18 (1⋵) | ||
| CHILE | 8(2⋵) | 1 | (3/)3 | (4/)5 (1⋵) | (9/)17 (3⋵) | ||
| ARGENTINA | 10(2⋵) | 2 | (3/)3 | (4/)6 | (9/)21(2⋵) | ||
| BRAZIL | 1 | 7(2⋵) | 6 | 1 | (2/)8 (3⋵) | (6/)23(5⋵) | |
| URUGUAY | 1 | (1/)1 | |||||
| PARAGUAY | 1 | (1/)1 | |||||
| SOUTH AMERICA | 12 | 34 | 7 | 2 | 3 | 29 | 87 |
87 spp. of Gymnosperms occurs in South America in 13 genera, nearly 1/3 palm-like cycads, 1/3 tropical montane trees podocarpids (sometimes shrubby or very tall tress - both groups with endemic species in Brazil) - and remaining as liana rainforests Gnetum, xeric to psamophyllous scandent or shrubby Ephedra, and trees of woody-pinids (Araucariaceae, Cupressaceae), none of them with endemic species in Brazil. Brazil has endemic species in three genera (Zamia, Retrophyllum and Podocarpus), and Bolivia in two; all others countries, only in a single. At family, Brazil has cycads (Zamiaceae) and conifers (Podocarpaceae) endemics; all other has endemics in a single family.
A. CYCADOPSIDA
Two families worldwide, Zamicaeae widely in tropical areas from New World, Africa and Australia, and Cycadaceae, with a single genus, Cycas L. (117), from Kenya to Mozambique, Madagascar, and from India to Japan, Fiji and Australia.
A1. ZAMIACEAE ‣ 253 spp. in nine genera: Encephalartos Lehm. (65, tropical Africa, from Ghana to Kenya, southern up to South Africa), Stangeria T.Moore (1, South Africa), Lepidozamia Regel (2, New South Wales, Australia), Bowenia Hook. (2, Queensland), Macrozamia Miq. (41, W, N & E Australia), Ceratozamia Brongn. (38, Mexico to Honduras), Dioon Lindl. (18, Mexico to Honduras), Microcycas (Miq.) A.DC. (1, Cuba) and Zamia L. (85, Mexico to C Brazil, up to Venezuela, Caribbean).
Zamia L. Perennial, evergreen, dioecious, stems subterranean with exposed apex or aboveground, fleshy, stout, cylindric, simple or irregularly branched; leaves pinnately compound, spirally clustered at stem apex, leathery; leaflets entire, dentate or spinose, venation dichotomous or netted; cones axillary, appearing terminal, short-peduncled or sessile, disintegrating at maturity; seed cones persisting a year or more, 1(2) per plant, nearly globose to ovoid; seeds angular, inner coat hardened, outer coat fleshy, often brightly colored; cotyledons.
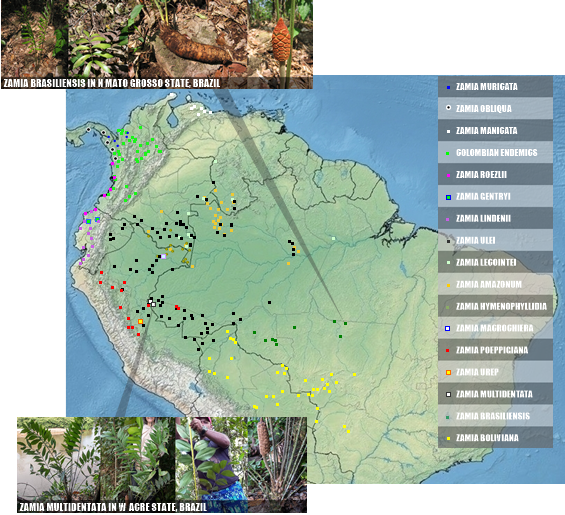
85 spp. from tropical America (POWO in September 28, 2024), 34 spp. in South America. 51 spp. does not occur in South America, in Mexico (17, 13⋵), Panamá (17, 12⋵, two up to Colombia), Cuba (6, 2⋵), Honduras (5, 3⋵), Guatemala (5, 2⋵), Costa Rica (5, 1⋵), Belize (4, 2⋵), Porto Rico (3, 1⋵), Bahamas (3, 1⋵), Jamaica (2), U.S.A., Cayman Is., Nicaragua and Rep. Dominicana one each.
Brazil has the 5ª diversity in New World, after Colombia (26), Mexico (17), Panamá (17) and Peru (8), with 7 spp.: Z. amazonum D.W. Stev. from Venezuela to Peru and Brazil; Z. boliviana (Brongn.) A. DC. from Bolivia to Mato Grosso state; Z. brasiliensis R. Segalla & Calonje from E Rondonia to NW Mato Grosso states (Segalla & Calonje, (Phytotaxa, 2019), Z. lecointei Ducke from Venezuela, Colombia, Peru and Brazil; Z. poeppigiana Mart. & Eichler from N & C Peru to Acre state in Brazil; Z. ulei Dammer from Venezuela to Bolivia and Brazil; and Z. multidentata Calonje, Segalla & R.S.Pimenta from W Acre state (Segalla et al., Phytotaxa, 2023), near Peruvian border.
Z. pseudoparasitica J.Yates in B.Seemann from Panamá is the only known species in this genus that is epiphytic, growing on the branches of forest trees (Wikipedia). A high remarkable beetle from Brazil (and Bolivia) is the Pharaxonotha cerradensis (Erotylidae), highly associated with Zamia boliviana (Brongniart) A. DC. (Cycadales: Zamiaceae), by Skelley & Segalla (Zootaxa, 2019). Additional data available on Segalla, R. et al. (Tropical Conservation Science, 2019). For Colombian Zamia, see Biovirtual/Flora de Colombia. For other work with details, see Colombian Zamia.
Zamia amazonum D.W.Stev., Fl. Colombia 21: 33 (2001).
S Venezuela to Peru. 82 VEN 83 CLM ECU PER 84 BZN. Cham.
DETAILED DESCRIPTION
Zamia amplifolia Mast., Gard. Chron., n.s., 10: 810 (1878).
NW Colombia. 83 CLM. Cham.
Zamia boliviana (Brongn.) A.DC. in A.P.de Candolle, Prodr. 16(2): 540 (1868).
Brazil (Mato Grosso) to N Bolivia. 83 BOL 84 BZC. Cham.
*Ceratozamia boliviana Brongn.
Zamia brasiliensis Calonje & Segalla, Phytotaxa 404: 4 (2019).
Brazil (Rondônia, Mato Grosso). 84 BZC BZN.
Zamia chigua Seem., Bot. Voy. Herald: 201 (1854).
W Colombia. 83 CLM. Cham.
Zamia disodon D.W.Stev. & Sabato, Fl. Colombia 21: 38 (2001).
Colombia. 83 CLM. Cham.
Zamia encephalartoides D.W.Stev., Fl. Colombia 21: 40 (2001).
NE Colombia. 83 CLM. Cham.
Zamia gentryi Dodson, Novon 8: 12 (1998).
Ecuador. 83 ECU. Cham.
Zamia huilensis Calonje, H.E.Esquivel & D.W.Stev., Caldasia 34: 284 (2012).
Colombia. 83 CLM.
Zamia hymenophyllidia D.W.Stev., Fl. Colombia 21: 43 (2001).
SE Colombia to N Peru. 83 CLM PER. Cham.
Zamia imbricata Calonje & J.Castro, Phytotaxa 497: 1 (2021).
Colombia. 83 CLM.
Zamia incognita A.Lindstr. & Idarraga, Phytotaxa 2: 30 (2009).
Colombia. 83 CLM. Cham.
Zamia lecointei Ducke, Arch. Jard. Bot. Rio de Janeiro 1: 9 (1915).
S Venezuela to N Peru, Brazil (Pará). 82 VEN 83 CLM PER 84 BZN. Cham.
HISTORY
Zamia lindenii Regel ex André, Ill. Hort. 22: 23, t. 195 (1875).
W Ecuador to NW Peru. 83 ECU PER. Nanophan. or phan.
MAP
Zamia lindosensis D.W.Stev., D.Cárdenas & N.Castaño, Brittonia 70: 364 (2018).
Colombia. 83 CLM.
Zamia macrochiera D.W.Stev., Cycad Classific. Concepts & Recommend.: 185 (2004).
N Peru. 83 PER. Cham.
Zamia manicata Linden ex Regel, Trudy Imp. S.-Peterburgsk. Bot. Sada 4: 310 (1876).
S Panama to NW Colombia. 80 PAN 83 CLM. Cham.
Zamia melanorrhachis D.W.Stev., Fl. Colombia 21: 55 (2001).
Colombia. 83 CLM. Cham.
Zamia montana A.Braun, Monatsber. Königl. Preuss. Akad. Wiss. Berlin 1875: 376 (1875).Colombia (Antioquia). 83 CLM. Cham. or nanophan.
:: Z. montana A.Braun is the highest elevation cycad in the world, found at 2,700m above sea level in Antioquia, Colombia (Palm Society).
Zamia multidentata Segalla, Pimenta & Calonje, 2023.
Brazil (Acre). 84 BZN.
Zamia muricata Willd., Sp. Pl., ed. 4. 4: 847 (1806).
Colombia to N Venezuela. 82 VEN 83 CLM. Cham.
Zamia obliqua A.Braun, Monatsber. Königl. Preuss. Akad. Wiss. Berlin 1875: 376 (1875).
S Panama to NW Colombia. 80 PAN 83 CLM. Cham. or nanophan.
Zamia oligodonta Calderón & D.W.Stev., Revista Acad. Colomb. Ci. Exact. 27: 486 (2003).
Colombia (Risaralda). 83 CLM. Hemicr. or cham.
Zamia orinoquiensis Calonje, Betancur & A.Lindstr., Phytotaxa 556: 124 (2022).
Colombia. 83 CLM.
Zamia paucifoliolata Calonje, Phytotaxa 385: 88 (2018).
Colombia. 83 CLM.
Zamia poeppigiana Mart. & Eichler in C.F.P.von Martius & auct. suc. (eds.), Fl. Bras. 4(1): 414 (1863).N & C Peru to Brazil (Acre). 83 PER 84 BZN. Nanophan.
MAP
Included in Colombia by Biovirtual/SEE, excluded by CZ/SEE and several other sources.
Zamia pyrophylla Calonje, D.W.Stev. & A.Lindstr., Brittonia 62: 80 (2010).
NW Colombia. 83 CLM. Cham.
Zamia restrepoi (D.W.Stev.) A.Lindstr., Taxon 58: 268 (2009).
NW Colombia. 83 CLM. Cham.
*Chigua restrepoi D.W.Stev.
Zamia roezlii Regel ex Linden, Cat. Gén. 90: 10 (1873).Colombia to Ecuador. 83 CLM ECU. Cham. or nanophan.
:: Z. roezlii Regel ex Linden, native to the rainforests of Colombia and Ecuador is the tallest of all Zamia species (Palm Society), and has the longest sperm produced by a plants, measuring approximately 0.4 mm long and is therefore visible to the naked eye (Guiness World Record).
Zamia sinuensis Calonje & J.Castro, Phytotaxa 497: 9 (2021).
Colombia. 83 CLM.
Zamia tolimensis Calonje, H.E.Esquivel & D.W.Stev., Brittonia 63: 443 (2011).
Colombia (Tolima). 83 CLM. Cham.
MAP
Zamia ulei Dammer, Verh. Bot. Vereins Prov. Brandenburg 48: 117 (1906 publ. 1907).
N South America. 82 VEN 83 BOL CLM ECU PER 84 BZN. Cham.
Zamia urep B.Walln., Linzer Biol. Beitr. 28: 1056 (1996).
Peru (Huánuco). 83 PER. Cham.
Zamia wallisii H.J.Veitch, Gard. Chron., n.s., 3: 795 (1875).
Colombia (Antioquia). 83 CLM. Cham.
GEOGRAPHICAL NOTES
18⋵ to Colombia (Z. amplifolia Mast., Z. chigua Seem., Z. disodon D.W.Stev. & Sabato, Z. encephalartoides D.W.Stev., Z. huilensis Calonje, Z. imbricata Calonje & J.Castro, Z. incognita A.Lindstr. & Idarraga, Z. lindosensis D.W.Stev., Z. melanorrhachis D.W.Stev., Z. montana A.Braun, Z. oligodonta Calderón & D.W.Stev., Z. orinoquiensis Calonje, Betancur & A.Lindstr., Z. paucifoliolata Calonje, Z. pyrophylla Calonje, Z. restrepoi (D.W.Stev.) A.Lindstr., Z. sinuensis Calonje & J.Castro, Z. tolimensis Calonje, H.E.Esquivel & D.W.Stev., Z. wallisii H.J.Veitch).Two from Colombia to Panamá (Z. manicata Linden ex Regel, Z. obliqua A.Braun).Z. muricata Willd., Z. roezlii Regel ex Linden and Z. hymenophyllidia D.W.Stev. from Colombia up to Venezuela, Ecuador and Peru, respectively.Three widely in northern South America, Z. amazonum D.W.Stev., Zamia lecointei Ducke and Z. ulei Dammer, all from Brazil, Peru, Colombia, Venezuela and Peru, sometimes up to Ecuador and Bolivia.Endemics in Ecuador (Z. gentryi Dodson), Peru (Z. macrochiera D.W.Stev., Z. urep B.Walln.) and Brazil (Z. multidentata Calonje, Segalla & R.S.Pimenta, Z. brasiliensis Calonje & Segalla).Three locally absents in Colombia: Z. lindenii Regel ex André in Peru and Ecuador; Z. poeppigiana Mart. & Eichler from Brazil and Peru; and Z. boliviana (Brongn.) A.DC. in Brazil and Bolivia.
The map below has been edited following the information analyzed in the post Revision of Podocarpus, Zamia and Brassicaceae in Brazil (LINK), from this same blog, for the Brazilian species. For the others, we follow GBIF or the sources mentioned below each name in the list above.
All analyses recovered the same broad topology consisting of the following geographically delimited major five clades: (1) Caribbean clade, consisting mostly of Caribbean Island species (a single species, Z. integrifolia, also reaches Florida), which is sister to the rest of the genus consisting of primarily of mainland American species; (2) Fischeri clade, consisting of three Mexican endemic species and is itself sister to the rest of the genus excluding the Caribbean clade; (3) Mesoamerica clade, including all other species occurring in Mesoamerica to the exclusion of the Fischeri clade and Z. soconuscensis; (4) Isthmus clade, consisting primarily of Panamanian and Costa Rican species; and (5) South America clade, consisting primarily of South American endemic species (Calonje et al., International Journal of Plant Science, 2019).
B. PINOPSIDA
B.1 GNETHIDAE/GNETHACEAE ‣ a single foresty genus.
Gnetum L. Dioecious, evergreen, mostly woody vines, rarely shrubs or trees; stems with swollen nodes; leaves opposite, petiolate, without stipules, simple, elliptic, with pinnate veins and entire margins; usually with drip tips; both male and female megastrobili terminal or lateral, sometimes arranged in dense, cauliflorous clusters on old stems; male strobilus consists of a stamen and perianth, the female strobilus of an ovule with 2 integuments and perianth (these structures are usually associated with angiosperms, one of the points that traditionally places Gnetum in an ambiguous state intermediate between the gymnosperms and angiosperms); seeds drupelike, enclosed in a red, orange, or yellow, fleshy (rarely corky) false seed coat. The wood contain tracheids and is otherwise typical of gymnosperms, but also contains vessels, another angiosperm trait, thought in this case to have arisen independently, without phylogenetic significance.
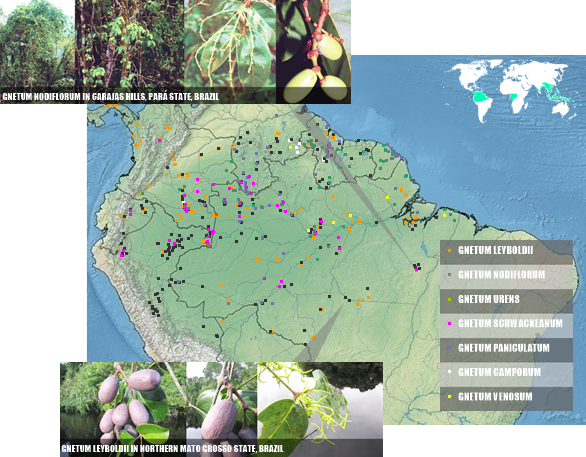
44 spp. worldwide, from Nigeria to Angola (4), SE China and Assan to Indo-China, Philippines and Sumatra to Queensland and Vanuatu (33) and from Costa Rica to Bolivia and N Brazil (7). South American species of Gnetum being sister to the remaining species. The four African species constitute a monophyletic group, sister to an Asian clade, within which the two arborescent species of the genus are the earliest diverging. New World species:
Gnetum nodiflorum Brongn. in L.I.Duperrey, Voy. Monde, Phan.: 12 (1829).
S. Trop. America. 82 FRG GUY SUR VEN 83 BOL CLM ECU PER 84 BZN. Cl.
Gnetum paniculatum Spruce ex Benth., Hooker's J. Bot. Kew Gard. Misc. 8: 357 (1856).
SE Colombia to N Brazil. 82 FRG GUY VEN 83 CLM 84 BZN. Cl. Also in Bolivia and Suriname by GBIF (SEE).
Gnetum schwackeanum Taub. ex Schenck, Beitr. Biol. Anat. Lianen 2: 249 (1893).
Colombia to Venezuela (Amazonas) and N Brazil. GUY 82 VEN 83 CLM 84 BZN. Cl. Also in Peru and Ecuador by GBIF (SEE).
Gnetum urens (Aubl.) Blume, Tijdschr. Natuurl. Gesch. Physiol. 1: 162 (1834).
Tropical South America. 82 FRG GUY SUR VEN 83 CLM PER 84 BZN. Cl.
*Thoa urens Aubl.
Gnetum venosum Spruce ex Benth., Hooker's J. Bot. Kew Gard. Misc. 8: 358 (1856).
Venezuela (Bolívar) to N Brazil. 82 VEN 84 BZN. Cl.
Gnetum camporum (Markgr.) D.W.Stev. & Zanoni, Fl. Guianas, Ser. A, 9(209): 14 (1991).
S Colombia, S Venezuela to Guyana. 82 GUY VEN 83 CLM. Cl.
*Gnetum urens var. camporum Markgr.
Gnetum leyboldii Tul., Ann. Sci. Nat., Bot., sér. 4, 10: 115 (1858).
Costa Rica to tropical South America. 80 COS PAN 82 VEN 83 BOL CLM ECU PER 84 BZN. Cl. Also in Guyana by GBIF (SEE).
Many distribution conflicts affect the data for Colombia, Venezuela and Guyana, with impacts for G. urens, often cited for Guyana (SEE), G. venosum rejected (SEE) or accepted (SEE) for Venezuela, G. camporum endemic (SEE) or no (SEE) from Venezuela. In view of this, the map provided below is the most consensual based on the references cited above and quite reliable for the Gnetum records by GBIF for G. nodiflorum, G. leyboldii, G. paniculatum and G. schwackeanum; GBIF also used for G. camporum (except for Brazilian record, possibly a mistake) and G. urens (except records from Central America, W Colombia, Ecuador and Peru, plus records for Brazil after Cavalcante in Acta Amazonica, 1978; Mota & Giulietti in Rodriguésia, 2016; and Zappi et al. in Acta Amazonica, 2011); finally, for G. venosum, we follows exclusivelly Cavalcante (Acta Amazonica, 1978), Mota & Giulietti (Rodriguésia, 2016) and Zappi et al. (Acta Amazonica, 2011).
B.2 GNETHIDAE/EPHEDRACEAE ‣ a single xeric genus.
Ephedra Tourn. ex. L. Perennial, dioecious (rarely monoecious), erect, procumbent or climbing shrubs or vines; bark grey to reddish brown, cracked and fissured, often fibrous; branching is often broom-like with nearly parallel and fastigiate to ascending green stems; branches photosynthetic, yellowish green to olive-green when young, round, finely longitudinally grooved, jointed, internodes 1-10 cm; leaves mostly not photosynthetic; vessels most abundant and largest in lianoid species, whilst nearly lacking in alpine species.
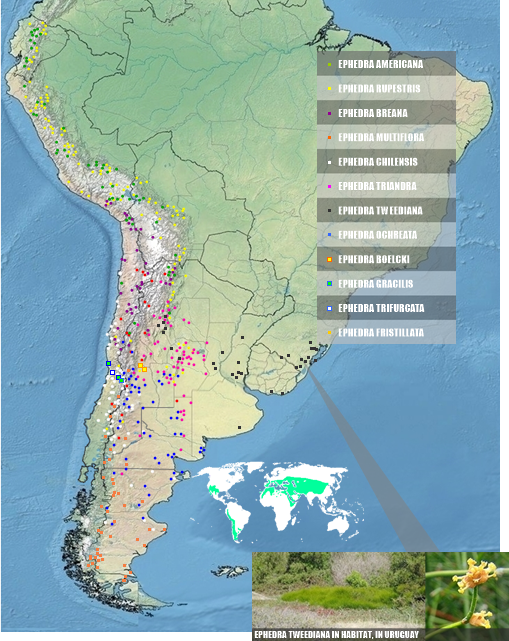
71 spp. worldwide, 12 in South America, 11 of them from western flank of continent, and E. tweedieana C.A.Mey. from SE coast of Rio Grande do Sul state in southern Brazil to N Argentina and S Uruguay.
Ephedra americana Humb. & Bonpl. ex Willd., Sp. Pl., ed. 4. 4: 860 (1806).
Ecuador to NW Argentina. 83 BOL ECU PER 85 AGW CLN. Cham.
Ephedra boelckei F.A.Roig, Parodiana 3: 11 (1984).
NW Argentina. 85 AGW.
Ephedra breana Phil., Anales Univ. Chile 91: 519 (1895).
SE Peru to NW Argentina. 83 BOL PER 85 AGW CLN. Cham. or nanophan.
Ephedra chilensis C.Presl, Abh. Königl. Böhm. Ges. Wiss., ser. 5, 3: 539 (1845).
C & S Chile to W Argentina. 85 AGS AGW CLC CLS. Cham. or nanophan.
Ephedra frustillata Miers, Ann. Mag. Nat. Hist., ser. 3, 11: 262 (1863).
C & S Chile to S Argentina. 85 AGS CLC CLS. Cham.
Ephedra gracilis Phil. ex Stapf, Denkschr. Kaiserl. Akad. Wiss., Wien. Math.-Naturwiss. Kl. 56(2): 87 (1889).
Chile (Atacama, Coqimbo). 85 CLC CLN. Cham. or nanophan.
Ephedra multiflora Phil. ex Stapf, Denkschr. Kaiserl. Akad. Wiss., Wien. Math.-Naturwiss. Kl. 52: 43 (1887).
Chile (Antofagasta), Argentina (Catamarca to Neuquén). 85 AGS AGW CLN. Nanophan.
Ephedra ochreata Miers, Ann. Mag. Nat. Hist., ser. 3, 11: 257 (1863).
Argentina (Catamarca to Santa Cruz). 85 AGS AGW. Cham. or nanophan.
Ephedra rupestris Benth., Pl. Hartw.: 253 (1846).
Ecuador to NW Argentina. 83 BOL ECU PER 85 AGW.
Ephedra triandra Tul., Ann. Sci. Nat., Bot., sér. 4, 10: 125 (1858).
Bolivia to Argentina. 83 BOL 85 AGE AGS AGW. Nanophan.
Ephedra trifurcata Zöllner, Anales Mus. Hist. Nat. Valparaiso 8: 81 (1975).
Chile (Valparaíso). 85 CLC. Nanophan.
Ephedra tweedieana C.A.Mey., Bull. Cl. Phys.-Math. Acad. Imp. Sci. Saint-Pétersbourg 5: 36 (1845).
S Brazil to NE Argentina. 84 BZS 85 AGE URU.
PHYLOGENY
This genus has 5 clades; among the Old World species, three highly-supported monophyletic groups are recognized, that are highly concordant with morphological evidence. The New World clade includes two main subclades of North and South American species that are strongly supported, while the position of two, mostly Mexican species E. pedunculata and E. compacta remains unresolved. Although all South American species belong to one highly-supported clade, relationships among these species are not well resolved, except the clade including E. tweediana, E. triandra, and E. chilensis.
A lianoid habit is reconstructed as having been derived in three different clades, in the Old World Fragilis group, in the North American E. pedunculata and in the South American clade with E. tweediana, E. triandra (Ickert-Bond & Wojciechowski, Systematic Botany, 2004).
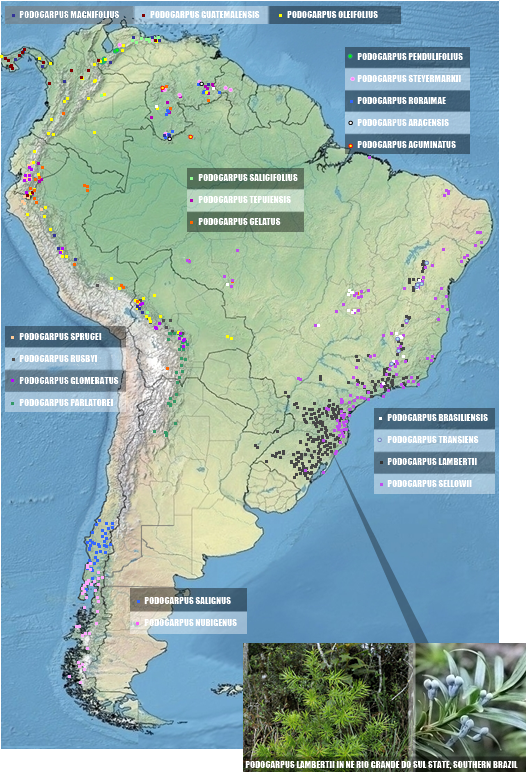
B.3 CUPRESSIDAE/PODOCARPACEAE ‣ remarkable data on the phylogeny of Podocarpaceae can be visited at Biffin et al. (Proceedings of The Royal Society, 2012). Six genera and 29 spp. in South America, 3/4 only Podocarpus. Map below based on Farjon (BOOK, 2013) except Retrophyllum, based on Mill, R.R. (Edinburgh Journal of Botany, 2016).
Three spp., two in New Zealand and L. fonkii Phil. in S Chile to SW Argentina.
Six spp., 3 in Australia, New Zealand and New Caledonia; P. standleyi (J.Buchholz & N.E.Gray) C.N.Page from Costa Rica, P. exigua (de Laub.) C.N.Page endemic to Bolivia, and P. harmsiana (Pilg.) C.N.Page from Venezuela to Bolivia.
Here we follow Farjon (Book, 2013) as the main reference, with 21 spp. on South America. 11 other species occur in remaining New World (Mexico, Central America and Caribbean). All South American members are restricted of continent except P. guatemalensis Standl. and P. oleifolius D. Don ex Lamb. which reaches up to Mexico, and P. magnifolius J. Buchholz & N.E. Gray up to Panamá.
The species in POWO in September 29, 2024, and Farjon (2013) are the same, differing only in the distributions of four: P. celatus, P. guatemalensis, P. parlatorei and P. tepuiensis. Here we accept all additions placed on P. celatus (Ecuador), P. guatemalensis (Venezuela and Ecuador) and in P. tepuiensis (Guyana). Here we reject the citation of P. parlatorei in Peru, in alignment with Frajon (2013).
BRAZIL
POWO cites 8 spp. in Brazil (P. acuminatus, P. aracensis, P. brasiliensis, P. celatus, P. lambertii, P. salicifolius, P. sellowii and P. transiens, the two lasts endemics) and three possibly native but not counted here, all from Monte Neblina (P. magnifolius, P. roraimae and P. steyermarkii).
Reflora also recognizes 8 native species from Brazil, 6 of which also in POWO (P. acuminatus, P. aracensis, P. brasiliensis, P. celatus, P. lambertii, P. sellowii, with the exception of P. salificolius and P. transiens) and includes two news, P. barretoi and P. roraimae.
We will discuss here the 12 spp. in a general sense mentioned/possibly mentioned for the country:
▪ P. magnifolius, P. roraimae, and P. steyermarkii are fully rejected here as Brazilian natives.
▪ P. salicifolius is rejected here for Brazil, unlike POWO, due to absence of consistent records for Reflora, TGD (The Gymnosperm Database), Farjon (2013) and Species Link.
▪ P. acuminatus and P. aracensis as the all sources, fully consistent except for a bizarre record from Goias cited in Species Link, rejected here.
▪ for P. celatus, out of the six main references, TGD and POWO seem to follow Farjon's map (2013), which marks the species only for Rondônia and Goiás. The occurrence in Goiás is strongly supported by Farjon's own text (2013), with these three references being stabilized. GBIF, Species Link, and Farjon (2013/MAP) are highly consistent in the occurrence of the species in Pacaas Novos in Rondônia, without contradictions. Mato Grosso is mentioned in Farjon's text (2013) without mapping, mentioned generically in POWO and Reflora for Mato Grosso, and not mentioned in GBIF. Thus, its validation in Mato Grosso is based solely on what is registered in Species Link: a record from 1961, 35 km from Vilhena. Based on these texts, we consider here that P. celatus occurs in three points in Brazil: Pacaas Novos (RO), NW Mato Grosso, and northern Goiás.
▪ P. transiens is not recognized in Reflora or in L.C. et al. In GBIF, Farjon (2013), Species Link, and TGD, it is recognized for both Bahia and Minas Gerais. Only Farjon (2013) and TGD recognize Goiás, Paraná, and Santa Catarina, and therefore, these records are rejected here.
▪ for P. lambertii, the non-mapped sources Reflora and TGD, the partially mapped L.C. Marinho et al. (Sitientibus, 2016) and the mapped GBIF are consistent from range from Bahia to Rio Grande do Sul also Minas Gerais, except form Espirito Santo, recognized only in Reflora and L.C. Marinho (Sitientibus, 2016). In Farjon (2013), P. lambertii has a narrower distribution, only for São Paulo, Paraná, Santa Catarina and Rio Grande do Sul, and border of Minas Gerais. Records from Species Link are highly consistent with all previous data except for not including Espírito Santo, expanding well beyond Farjon (2013), and including records in Mato Grosso do Sul (a single records in Iguatemi), Goiás (5, 4 in Trindade and one in Alto Paraiso de Goias), and the Distrito Federal (3 very inconclusive records). For the map below, we follow the data from Farjon (2013), GBIF, and Species Link, excluding the records from Espírito Santo, Goiás and the Distrito Federal.
▪ Podocarpus brasiliensis is very conflitant. Out of the 6 main references, there is consensus only for Goiás, the Distrito Federal, and Mato Grosso. Farjon (2013), GBIF, and Species Link provide good options for pinpoint records. Farjon (2013) and TGD exclude Paraná and include Roraima, although Farjon (2013) does not map it. São Paulo is only mentioned by Species Link for a record in Guaratinguetá; Amazonas is mentioned only for Species Link for NW region; and Rondônia is only mentioned by Farjon (2013) with a record in Pacaas Novos. Bahia is mentioned by all except Reflora and L.C. Marinho (Sitientibus, 2016). Bahia, Minas Gerais, Roraima, Paraná, São Paulo and Rondonia are the conflitant states.
The record from São Paulo in Guaratinguetá is accepted here.
The record in Rondônia, indicated only by Farjon (2013) and TGD, is rejected here.
The record in Paraná, the same in Species Link and GBIF, in Piraquara, was previously considered P. sellowi, changed to P. brasiliensis in 1988 by E. Cope. Due to the extreme inconsistency of the standard distribution, we consider it as P. sellowi here.
The record in Amazonas, cited only in Species Link, is rejected here due to its extreme inconsistency with the other records of the species.
The record in Roraima is rejected here because it was only cited in Farjon (2013) and TGD, and it wasn't even mapped in the first reference.
Here we accept the records from Minas Gerais, a mix of GBIF, Species Link, and Farjon, relatively consistent, plus cites of P. barretoi.
For Bahia, we also accept here a mix of GBIF, Species Link, and Farjon, despite the exclusion of this species in L.C. Marinho (Sitientibus, 2016).
▪ P. sellowii as in Farjon (2013), and following notes. Records from Maranhão in GBIF ans Species is rejected by does not be Podocarpacaeae, but Amaranthaceae. Records in Ceara follows a mixed from GBIF and Species Link except cultivated records. The GBIF and Species Link records from Pernambuco accepted are only those from Caruaru, as the records from Recife are of cultivated material, and those from Rio Formoso belong to Conocarpus erectus. The records from Alagoas and Sergipe are fully accepted as GBIF and Species Link. Records in GBIF and Species Link from Bahia to Rio Grande do Sul are fully accepted here except records in Salvador City.
In these terms, we confirm 7 species in Brazil here. National diversities, from greatest to least, are: Venezuela (12, 1⋵), Brazil (7, 2⋵), Bolivia (7), Peru (7), Ecuador (6), Colombia (5), Argentina (3), Guyana (3), Chile (2, 1⋵). Podocarpaceae in Brazil needs a urgent revision!
CHECKLIST OF SOUTH AMERICAN SPECIES
Podocarpus acuminatus de Laub., Novon 2: 329 (1992).S Venezuela to N Brazil (Serra da Neblina). 82 VEN 84 BZN. Phan.
Podocarpus aracensis de Laub. & Silba, Phytologia 65: 330 (1988).S Venezuela (Cerro Yaví) to N Brazil (Serra Araca). 82 VEN 84 BZN. Phan.
Podocarpus brasiliensis de Laub., Fl. Venez. 11(2): 31 (1982).Venezuela to N & C Brazil. 82 VEN 84 BZC BZL BZN. Phan.
Podocarpus celatus de Laub., Fl. Venez. 11(2): 35 (1982).S Venezuela to Bolivia. 82 VEN 83 BOL CLM PER 84 BZC. Phan.
Podocarpus glomeratus D.Don in A.B.Lambert, Descr. Pinus 2: 21 (1824).Ecuador to Bolivia. 83 BOL ECU PER. Phan.Podocarpus guatemalensis Standl., Proc. Biol. Soc. Washington 32: 49 (1924).Mexico (Veracruz, Oaxaca) to Colombia. 79 MXG MXS 80 BLZ COS GUA HON NIC PAN 82 VEN? 83 CLM. Phan.
Cited for Venezuela and Ecuador by Farjon (2013).
Podocarpus lambertii Klotzsch ex Endl., Syn. Conif.: 211 (1847).SE & S Brazil to Argentina (Misiones). 84 BZL BZS 85 AGE. Phan.
Podocarpus magnifolius J.Buchholz & N.E.Gray, J. Arnold Arbor. 29: 133 (1948).Panama to W South America and Venezuela. 80 PAN 82 VEN 83 BOL CLM PER. Phan.
Possibly in Brazilian side of Neblina Massif by Farjon (2013) and Guyana by Gymnosperms Database (SEE).
Podocarpus nubigenus Lindl., J. Hort. Soc. London 6: 264 (1851).S Chile to S Argentina. 85 AGS CLS. Phan.Podocarpus oleifolius D.Don in A.B.Lambert, Descr. Pinus 2: 20 (1824).S Mexico to W. South America. 79 MXG MXS MXT 80 COS ELS GUA HON NIC PAN 82 VEN 83 BOL CLM ECU PER. Phan.
Podocarpus oleifolius subsp. oleifolius.
W. South America to Venezuela. 82 VEN 83 BOL CLM ECU PER. Phan.
Podocarpus parlatorei Pilg. in H.G.A.Engler (ed.), Pflanzenr., IV, 5: 86 (1903).Peru to NW Argentina. 83 BOL PER 85 AGW. Phan.* Podocarpus angustifolius Parl.
Absent in Peru in Farjon (2013).
Podocarpus pendulifolius J.Buchholz & N.E.Gray, J. Arnold Arbor. 29: 138 (1948).NW Venezuela. 82 VEN. Phan.Podocarpus roraimae Pilg., Notizbl. Königl. Bot. Gart. Berlin 5: 299 (1913).S Venezuela to Guyana (Mt. Roraima). 82 GUY VEN. Phan.
Despite its occurrence in two tepuis shared by Brazil, there is no reliable record of its presence in the Brazilian part of these tepuis.
Cited in Brazil for Mainieri & Pires (Silvicultura em São Paulo, 1973).
Podocarpus rusbyi J.Buchholz & N.E.Gray, J. Arnold Arbor. 29: 134 (1948).S Peru to Bolivia. 83 BOL PER. Phan.Podocarpus salicifolius Klotzsch & H.Karst. ex Endl., Syn. Conif.: 209 (1847).Venezuela to W South America and N Brazil. 82 VEN 83 BOL CLM PER 84 BZN. Phan.
Not cited in Reflora.
Farjon (2013) not mapped none record in Brazil.
Podocarpus salignus D.Don in A.B.Lambert, Descr. Pinus 2: 20 (1824).SC & S. Chile 85 CLC CLS. Phan.
Fully absent in Argentina.
Podocarpus sellowii Klotzsch ex Endl., Syn. Conif.: 209 (1847).Brazil (Rio de Janeiro to Rio Grande do Sul). 84 BZL BZS. Phan.
Reflora cites this species in AC, PA, RO, AL, BA, CE, PE, SE, DF, GO, MT, MS, MG and ES, unlike Farjon (2013, mapped only in RS, SC, PR, SP, RJ) and TGD Link.
Garcia (FFES, 2016) recognizes in up to Pará and Rondonia states.
Mapped and cited in Bahia by L.C. Marinho et al. (Sitientibus, 2016).
Podocarpus sellowii var. angustifolius Pilg. in H.G.A.Engler (ed.), Pflanzenr., IV, 5: 88 (1903).Brazil (Rio de Janeiro). 84 BZL. Phan.
Two districts of Rio de Janeiro: Cerro dos Orgãos and Pico do Tingua.Podocarpus sellowii var. sellowii.Brazil (Rio de Janeiro to Rio Grande do Sul). 84 BZL BZS. Phan.
Podocarpus sprucei Parl. in A.P.de Candolle, Prodr. 16(2): 510 (1868).W Ecuador to N Peru. 83 ECU PER. Phan.Podocarpus steyermarkii J.Buchholz & N.E.Gray, J. Arnold Arbor. 29: 133 (1948).Venezuela to Guyana (Pakaraima Mts.). 82 GUY VEN. Phan.
Despite its occurrence in Venezulan side of Neblina Massif, there is no reliable record of its presence in the Brazilian part of this tepui.
Podocarpus tepuiensis J.Buchholz & N.E.Gray, J. Arnold Arbor. 29: 134 (1948).S. Venezuela, SE Ecuador. 82 VEN 83 ECU. Phan.
Cited for Guyana in Farjon (2013).
Podocarpus transiens (Pilg.) de Laub., Phytologia Mem. 7: 68 (1984).Brazil. 84 BZC BZE BZL BZS. Phan.*Podocarpus lambertii var. transiens Pilg.
Non cited in Reflora.
Non mapped neither cited in Bahia by L.C. Marinho et al. (Sitientibus, 2016).
Farjon (2013, mapped in GO, BA, MG) no mapped records in Paraná and Santa Catarina states, unlike TGD.
Three spp., one in New Zealand, P. andina (Poepp. ex Endl.) de Laub from SC & S Chile to Neuquén in Argentina, and P. montana (Humb. & Bonpl. ex Willd.) de Laub. from NW Venezuela to N Peru.
6 spp., four in Oceania, R. piresii (Silba) C.N.Page endemic to Serra dos Pacaás Novos, SW Rondônia state, N Brazil, and R. rospigliosii (Pilg.) C.N.Page from NW Venezuela to Bolivia. For a excellent revision of this genus, see Mill, R.R. (Edinburgh Journal of Botany, 2016).
In August 1976, on a trip undertaken by the late Dr. João Murça Pires, with William Antônio Rodrigues as his tour companion and the Botany parataxonomist, Nelson Rosas, they were in the Serra Pacaas Novos region, Rondônia, with the strategic support of a helicopter kindly provided by Projeto Radambrasil. In a clearing previously opened by Radam for prospecting its flora and ecological studies, in the middle of the forest located in Seringal São Luiz, 50 km east of the Pacaas Novos river, we managed to collect some exsiccates from a large tree (30 ✕ 0.60 m) of Gymnosperma, which Most of it (1983) was described as Decussocarpus piresii by Silba, with this name being used as a basionym for Nageria piresii (Silba) Laubeng in 1987 and recently Retrophyllum piresii (Silba) C. N. Page, in 1989. Only a single tree of the aforementioned species was found. species at the site, which was fruiting at the time. Some seeds were collected by Dr. Murça Pires, which, after germinating, were introduced into the Garden of the Emílio Goeldi Museum, in Belém, Pará. Two of these seedlings are growing well, currently measuring around 5m. tall, constituting a rare specimen of the species in question being cultivated outside its natural habitat. Due to the great deforestation that has been occurring lately in the indigenous area of Paacas Novos, Rondônia, this species is at great risk of extinction. The botanical samples are deposited, especially, in the herbaria of INPA, MG, and US (SEE ORIGINAL).
A single sp., S. conspicua Lindl., from SC & S Chile to S Argentina.
B.4 CUPRESSIDAE/ARAUCARIACEAE ‣ evergreen trees with spirally arranged, narrow or broad leaves often with parallel veins; dioecious or monoecious; male cones relatively large, cylindrical, with numerous sporophylls and with c. 12 inverted pollen sacs; pollen grains wingless; female cone usually borne erect, subglobose to ovoid, maturing in two years, relatively large and milky, falling upon maturity; scales one-seeded, without distinct bracts; cotyledons 4, often fused into 2 double cotyledons.
(3/)41 spp. in three genera, with Wollemia W.G.Jones, K.D.Hill & J.M.Allen (1) endemic to Australia, Agathis Salisb. (17) found in New Zealand, Australia, Vanuatu, New Caledonia, Papua New Guinea, Indonesia, Malaysia, and the Philippines, and Araucaria Juss. (20), from New Caledonia (14), one in Norfolk Island, three in Australia to New Guinea (one endemic each plus one shared), and two in South America.
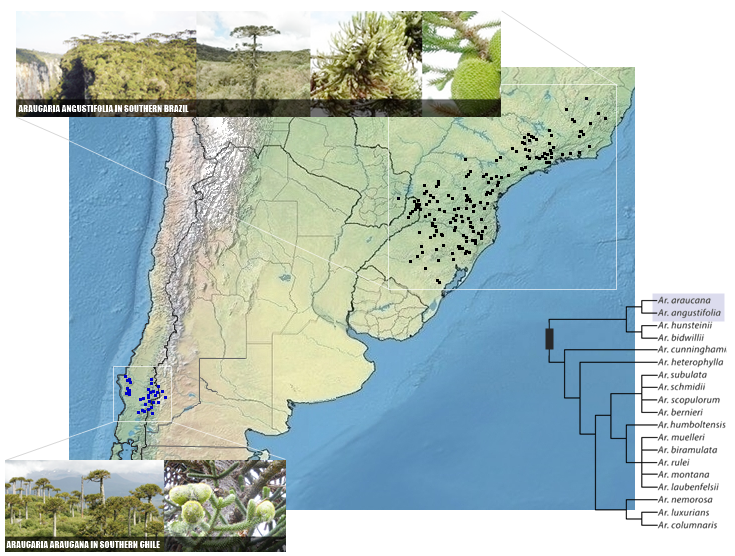
Araucaria angustifolia (Bertol.) Kuntze, Revis. Gen. Pl. 3(2): 375 (1898).
SE & S Brazil to Argentina (Misiones) and E Paraguay. 84 BZL BZS 85 AGE PAR. Phan.
*Columbea angustifolia Bertol.
Araucaria araucana (Molina) K.Koch, Dendrologie 2(2): 206 (1873).
SC Chile to Argentina (Neuquén). (10) grb 85 AGS CLC CLS. Phan.
*Pinus araucana Molina.
For A. angustifolia, map below follows Farjon (Book, 2013), Inza et al. (Trees, 2018, for Argentina), Silva, P.I.T. et al. (Plos One, 2020), Whege et al. (EMBRAPA, 2021), Species Link (partially, SEE), and Parques Nacionales de Paraguay (for Paraguay, SEE). For A. araucana, we follows only Farjon (Book, 2013).
B.5 CUPRESSIDAE/CUPRESSACEAE ‣ trees or shrubs, generally resinous and aromatic, monoecious (usually dioecious in Juniperus); lateral branches densely clothed by scalelike leaves or by decurrent leaf bases; longest internodes to 1 cm; buds undifferentiated and inconspicuous (except in Sequoia, Metasequoia, Cunninghamia and Juniperus sects. Juniperus and Caryocedrus); pollen cones maturing and shed annually, solitary, terminal (rarely in clusters of 2-5, or to 20 or more in Cunninghamia; axillary in Cryptomeria and Juniperus sects. Juniperus and Caryocedrus; in terminal panicles in Taxodium and Metasequoia), simple, spheric to oblong; sporophylls overlapping, bearing 2-10 abaxial microsporangia (pollen sacs); pollen spheric, not winged; seed cones maturing in 1-2 seasons, compound, solitary, terminal (rarely in clusters of 2-5, or up to 100 or more in Widdringtonia; axillary in Juniperus sects. Juniperus and Caryocedrus); seeds 1-20 per scale. All Cupressaceae appear to share a vesicular-arbuscular mycorrhiza.
A family with 30 genera and 167 spp., in six lineages, two most basal in Asia (2/3), the next two disjuncts in Japan, China, Laos, Vietnan, U.S.A., Mexico, Guatemala and Tasmania in Australia (7/8), and two super irradiations, Cupressoideae in northern Hemisphere (13/122, c. 3/5 of diversity in Juniperus), and Callitroideae in Australaisia, Africa and southern South America (8/34, 20 in Callitris). Three genera and species in South America, all from SC & S Chile to S Argentina.
Austrocedrus chilensis (D.Don) Pic.Serm. & Bizzarri, Webbia 32: 482 (1978).
C & SC Chile to SW. Argentina. 85 AGS CLC CLS. Phan.
*Thuja chilensis D.Don
Fitzroya cupressoides (Molina) I.M.Johnst., Contr. Gray Herb. 70: 91 (1924).
Chile (Los Lagos) to SW Argentina. 85 AGS CLS. Phan.
*Pinus cupressoides Molina
Pilgerodendron uviferum (D.Don) Florin, Svensk Bot. Tidskr. 24: 133 (1930).
S Chile to S Argentina. 85 AGS CLS. Phan.
*Juniperus uvifera D.Don